AstraZeneca’s Path to a Governed Data Marketplace
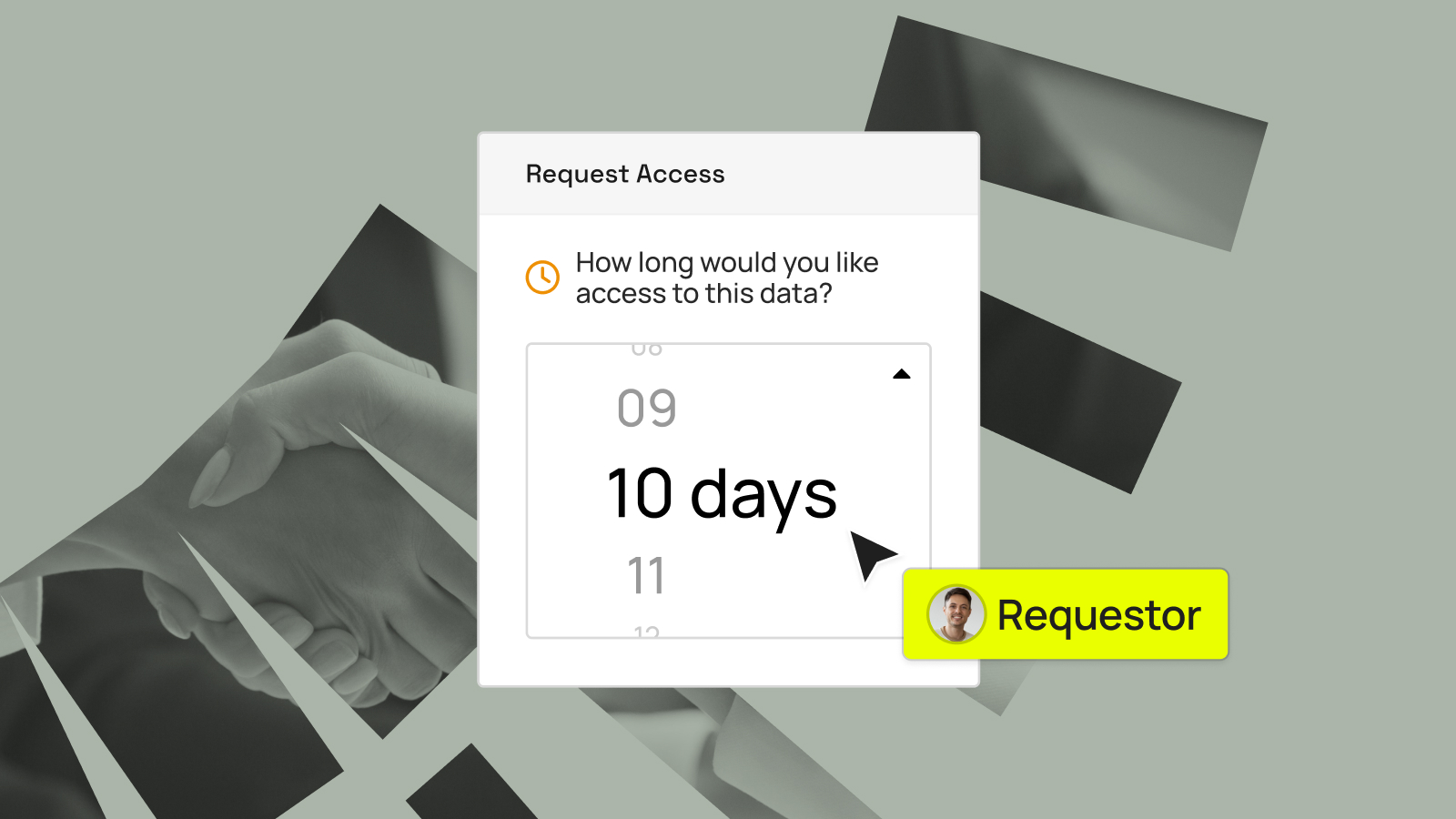
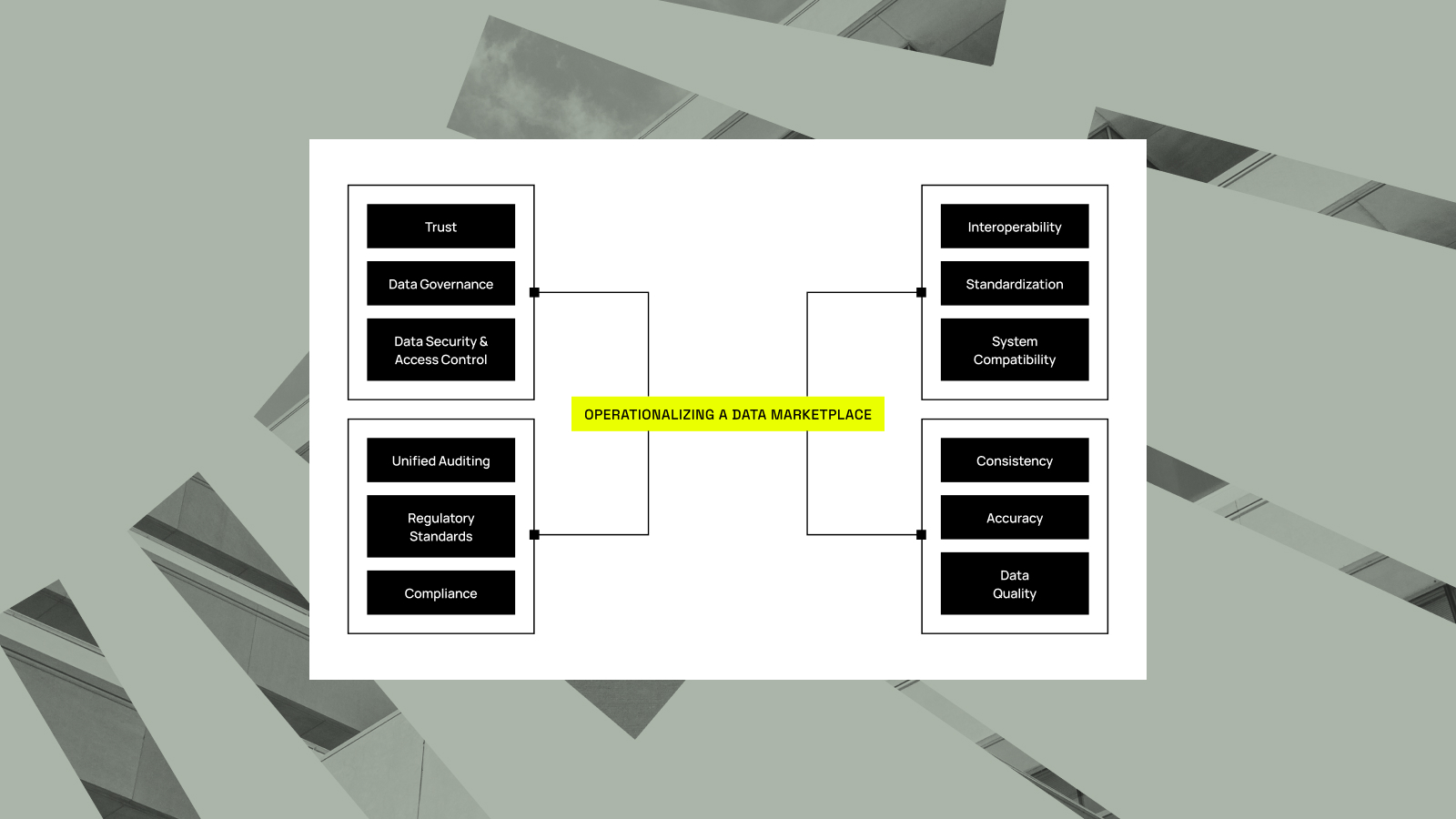
AstraZeneca’s mission is clear: unlock science for patients, responsibly. That commitment shows up in how they manage data, ensuring it’s “responsibly sourced, rapidly available, and always protected,” as Gayathri Ramamoorthy, Associate Director of Data Engineering at AstraZeneca, explained during a recent webinar. That standard is hard to meet when research...